A galvanic reaction occurs when two dissimilar metals are in electrical contact with one another which causes one metal to corrode. Noble metals (gold, silver, platinum, palladium) are resistant to corrosion and oxidation. When these metals are mated with more corrosive metals (tin), a Galvanic reaction would occur.
This is important to consider when adding components to printed circuitry, many components come tin plated. When a silver epoxy is laid down to mate components to the circuitry, a tin plated component would be a sure-fire way to risk a galvanic corrosion. Another downfall from this reaction is that it would also create non-conductive oxide at the joints, which would then lead to failed components.
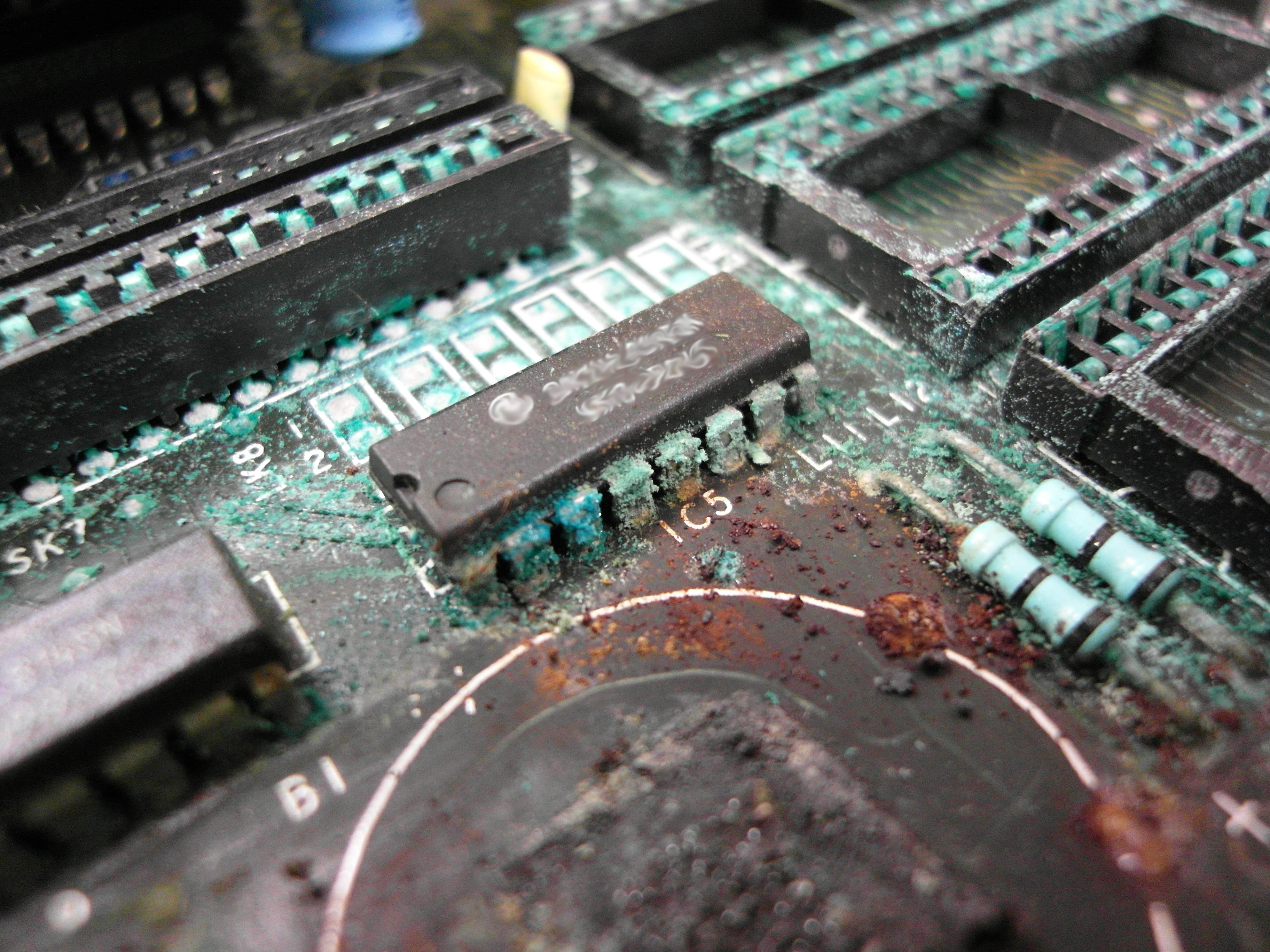
This is obviously an exaggerated picture but you want to avoid any and all corrosion & oxidization! (photo courtesy of Dave L. Jones (Binarysequence) on Creative Commons)
Using gold or silver plated components would prevent this reaction; a noble metal on the component contact and the noble metal in the silver epoxy. Two noble metals “play” together much nicer.
This would explain why many LEDs are gold plated! The plating on the LED’s has two important functions, electrical performance and the ability to “mate with the base circuit”. With the circuit board industry being a large (if not the largest) user of LEDs, and the conductive circuitry on most boards being a gold plating, the gold contacts on the LEDs mate extremely well to the gold traces.
Avoid gavanic reactions and component failure, work with an expert!
Manufacturing our conductive epoxy in-house gives us complete control of cost, volume, viscosity, and resistance. Pair that with intentionally selected components and we can effectively avoid unnecessary corrosion.
